Notas de prensa
- Política sanitaria
- General
CPhI Worldwide, closes its 29th edition with pre-audit figures showing the most international audience in the event’s history, with a total unique attendance of 44,500[1] – remarkably, over 39,000 of which were international.
CPhI Worldwide, closes its 29thedition with pre-audit figures showing the most international audience in the event’s history, with a total unique attendance of 44,500 – remarkably, over 39,000 of which were international.
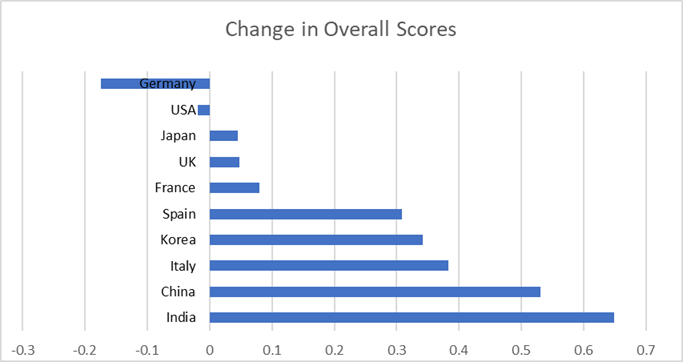
Emphasizing the strength of global pharma over the past year, in the annual survey conducted for CPhI Worldwide (CPhI Pharma Index), eight of ten top nations improved their overall score across all categories – with table topping nations the USA and Germany the only countries not to improve. In fact, for the second year running Germany, Japan and the United States emerged as the top-ranked, tier-one nations for pharmaceutical quality and overall score – with India and China making dramatic strides in raising their overall reputation. Host nation of CPhI Worldwide 2018, Spain, also made a significant improvement with international perception of ‘innovation emerging in the country’ rising – driven notably by the biotech hubs in both Barcelona and Madrid. However, most promising, was the improvement in growth potential industry-wide, as eight out of ten countries’ scores rose – with only Spain and China marginally lower than in 2017.



